Perchloric acid and potassium hydroxide net ionic equation – Delving into the realm of chemistry, we encounter the captivating reaction between perchloric acid and potassium hydroxide. This intriguing interaction unveils a symphony of chemical principles, showcasing the interplay of strong oxidizing and basic properties. Join us as we embark on a journey to decipher the net ionic equation of this reaction, unraveling its significance and applications.
Perchloric acid, a highly corrosive and powerful oxidizing agent, reacts with potassium hydroxide, a strong base, to yield a distinctive set of products. The net ionic equation, stripped of spectator ions, provides a concise representation of the core chemical transformation.
Understanding this equation empowers us to predict the reaction’s behavior, optimize experimental conditions, and harness its potential in diverse fields.
Perchloric Acid Properties
Perchloric acid (HClO 4) is a highly reactive inorganic acid with several unique properties.
Strong Oxidizing Properties
Perchloric acid is a powerful oxidizing agent due to the presence of the perchlorate ion (ClO 4–). It can readily accept electrons and undergo reduction to form chloride ions (Cl –).
Examples of its use as an oxidizer:
- In rocket fuels
- In the production of explosives
- As an etching agent in metalworking
Precautions when Handling Perchloric Acid
Perchloric acid is a corrosive and explosive substance. It must be handled with extreme caution, using appropriate protective gear and following strict safety protocols.
Potassium Hydroxide Properties
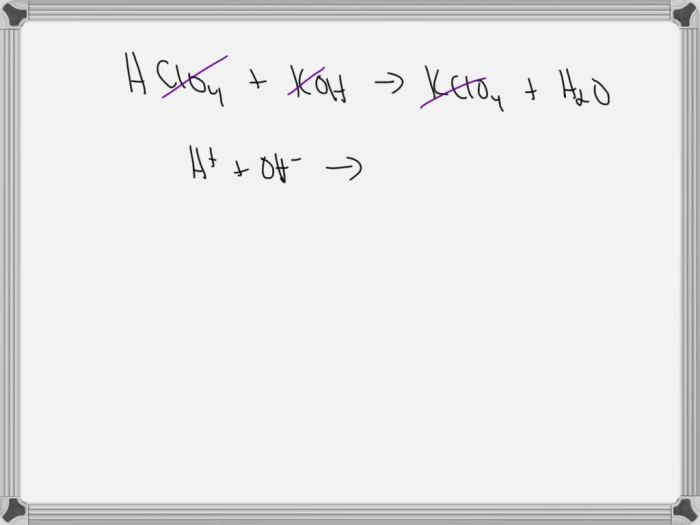
Potassium hydroxide (KOH) is a strong alkali with several important properties.
Basic Properties
Potassium hydroxide readily dissociates in water to form potassium ions (K +) and hydroxide ions (OH –). This dissociation makes it a strong base, capable of neutralizing acids and producing salts.
Examples of its use as a strong base:
- In the production of soap and detergents
- As a cleaning agent
- In the manufacture of fertilizers
Safety Considerations
Potassium hydroxide is corrosive and can cause severe burns. It must be handled with care, using appropriate protective gear and following proper safety procedures.
Net Ionic Equation
The balanced chemical equation for the reaction between perchloric acid and potassium hydroxide is:
HClO 4+ KOH → KCl + H 2O
The spectator ions in this reaction are potassium (K +) and chloride (Cl –) because they do not participate in the chemical change. The net ionic equation is:
H ++ OH –→ H 2O
Reaction Conditions
The reaction between perchloric acid and potassium hydroxide occurs rapidly at room temperature. The rate of reaction can be increased by heating the reactants or by increasing their concentrations.
Experimental Procedures:
- Add a known mass of perchloric acid to a flask.
- Add a known mass of potassium hydroxide to the flask.
- Heat the flask gently while stirring.
- Monitor the temperature and the reaction progress.
Reaction Products
The products of the reaction between perchloric acid and potassium hydroxide are potassium chloride (KCl) and water (H 2O).
Potassium Chloride, Perchloric acid and potassium hydroxide net ionic equation
Potassium chloride is a white, crystalline solid that is highly soluble in water. It is commonly used as a fertilizer and as a food additive.
Water
Water is a colorless, odorless, and tasteless liquid that is essential for life. It is used in a wide variety of applications, including drinking, irrigation, and industrial processes.
Applications

The reaction between perchloric acid and potassium hydroxide is used in various applications, including:
- Laboratory analysis:To neutralize acids and to prepare potassium chloride solutions.
- Industrial processes:To produce fertilizers, detergents, and other chemicals.
- Water treatment:To remove impurities and to adjust the pH of water.
Quick FAQs: Perchloric Acid And Potassium Hydroxide Net Ionic Equation
What safety precautions should be taken when handling perchloric acid?
Perchloric acid is highly corrosive and can cause severe burns. It should be handled with extreme caution, wearing appropriate protective gear, including gloves, goggles, and a lab coat. The reaction should be carried out in a well-ventilated area.
What are the applications of the reaction between perchloric acid and potassium hydroxide?
This reaction is used in various fields, including analytical chemistry, electrochemistry, and organic synthesis. It is also employed in the production of perchlorates, which are used in fireworks, explosives, and fertilizers.