Welcome to the world of gas laws! This comprehensive combined gas law worksheet and answers guide is designed to provide you with a thorough understanding of the combined gas law, its applications, and common pitfalls. Get ready to delve into the fascinating realm of gases and their behavior under varying conditions.
The combined gas law is a powerful tool that allows us to predict the behavior of gases under different conditions of pressure, volume, temperature, and number of moles. In this guide, we will explore the concept of the combined gas law, solve a variety of worksheet problems, and discuss its applications in chemistry and real-world scenarios.
Combined Gas Law Overview
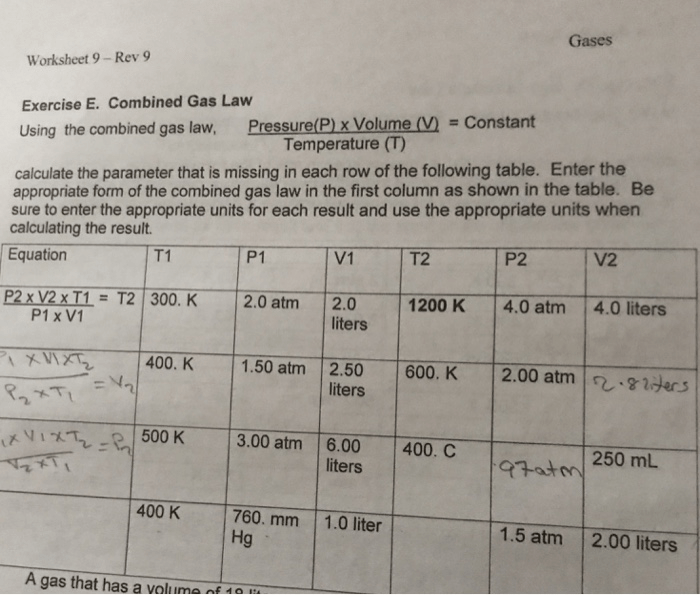
The combined gas law is a mathematical equation that describes the relationship between the pressure, volume, temperature, and number of moles of a gas. It is a combination of Boyle’s law, Charles’s law, and Gay-Lussac’s law. The combined gas law can be used to predict the behavior of gases under different conditions.
The combined gas law equation is: $$\fracP_1V_1T_1 = \fracP_2V_2T_2$$ where: – P is the pressure of the gas – V is the volume of the gas – T is the temperature of the gas – the subscripts 1 and 2 refer to the initial and final states of the gas
The combined gas law can be used to solve a variety of problems, such as:
- Predicting the pressure of a gas if its volume or temperature changes
- Predicting the volume of a gas if its pressure or temperature changes
- Predicting the temperature of a gas if its pressure or volume changes
- Calculating the number of moles of a gas if its pressure, volume, and temperature are known
FAQ Overview: Combined Gas Law Worksheet And Answers
What is the combined gas law?
The combined gas law is a combination of Boyle’s law, Charles’s law, and Gay-Lussac’s law. It relates the pressure, volume, temperature, and number of moles of a gas in a single equation.
How can I use the combined gas law to solve problems?
To use the combined gas law, you can rearrange the equation to solve for the unknown variable. Make sure to convert all units to SI units (Pascals for pressure, liters for volume, Kelvin for temperature, and moles for number of moles) before plugging them into the equation.
What are some common applications of the combined gas law?
The combined gas law has numerous applications in chemistry, including determining the molar mass of a gas, calculating the volume of a gas at different conditions, and predicting the behavior of gases in chemical reactions.