Arrange these complexes in order of octahedral splitting energy δo – Arrange these complexes in order of octahedral splitting energy (δo), a fundamental concept in inorganic chemistry. This energy plays a crucial role in determining the properties and reactivity of coordination complexes. By understanding the factors that influence δo, chemists can predict the behavior of these complexes and design new materials with tailored properties.
The magnitude of δo is primarily influenced by the nature of the ligands coordinated to the metal ion. Stronger ligands, such as those containing nitrogen or oxygen donor atoms, result in a larger δo. Additionally, the oxidation state of the metal ion and the number of d electrons can also affect the splitting energy.
Octahedral Splitting Energy (δo): Arrange These Complexes In Order Of Octahedral Splitting Energy δo
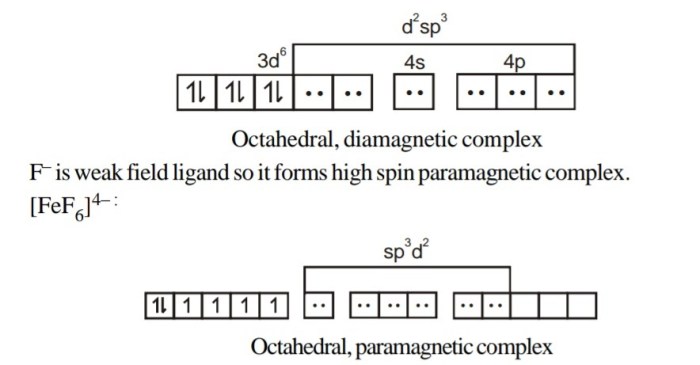
Octahedral splitting energy (δo) is the energy difference between the t2g and eg orbitals in an octahedral complex. The magnitude of δo is determined by the strength of the ligand field, which is in turn affected by the nature of the ligand and the metal ion.
Ligand Field Theory
Ligand field theory is a model that describes the bonding between metal ions and ligands. According to ligand field theory, the metal ion is surrounded by a cloud of electrons that are donated by the ligands. The ligands can be either strong-field or weak-field ligands.
Strong-field ligands cause a large splitting of the d orbitals, while weak-field ligands cause a small splitting.
Spectrochemical Series
The spectrochemical series is a list of ligands that are arranged in order of their ability to cause a splitting of the d orbitals. The ligands at the top of the series are strong-field ligands, while the ligands at the bottom of the series are weak-field ligands.
- CN-
- NO2-
- en
- NH3
- H2O
- F-
- Cl-
- Br-
- I-
Experimental Determination of δo, Arrange these complexes in order of octahedral splitting energy δo
There are a number of experimental methods that can be used to determine the octahedral splitting energy of a complex. These methods include:
- Electronic spectroscopy
- Magnetic susceptibility measurements
- X-ray crystallography
Each of these methods has its own advantages and disadvantages.
Applications of Octahedral Splitting Energy
Octahedral splitting energy is a useful tool for understanding the properties and reactivity of coordination complexes. For example, δo can be used to:
- Predict the color of a complex
- Determine the magnetic properties of a complex
- Understand the reactivity of a complex
User Queries
What is octahedral splitting energy (δo)?
Octahedral splitting energy is the energy difference between the t2g and eg orbitals in an octahedral complex. It arises from the interaction between the d electrons of the metal ion and the ligands coordinated to it.
How does the spectrochemical series help predict δo?
The spectrochemical series is a ranking of ligands based on their ability to cause octahedral splitting. Ligands that are higher in the series produce a larger δo.
What factors affect the magnitude of δo?
The magnitude of δo is primarily influenced by the nature of the ligands coordinated to the metal ion, as well as the oxidation state of the metal ion and the number of d electrons.