In the realm of chemistry, the complete hydrogenation of ethyne holds a captivating place. This process, meticulously described as “draw the product of the complete hydrogenation of ethyne,” unravels the fascinating transformation of a simple hydrocarbon into a more saturated counterpart.
Embark on this journey of discovery, where we delve into the intricate details of this reaction, its mechanisms, and its far-reaching applications.
The essence of hydrogenation lies in the addition of hydrogen molecules to unsaturated compounds, leading to the formation of more saturated compounds. When ethyne, a hydrocarbon characterized by a triple bond between its carbon atoms, undergoes complete hydrogenation, it undergoes a profound chemical transformation.
This process results in the conversion of ethyne into ethane, a saturated hydrocarbon with a single bond between its carbon atoms.
The Complete Hydrogenation of Ethyne: Draw The Product Of The Complete Hydrogenation Of Ethyne
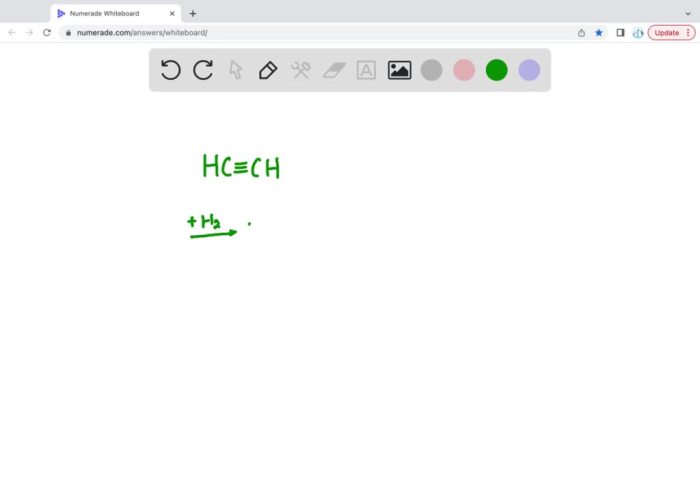
The complete hydrogenation of ethyne is a chemical reaction that converts ethyne (C 2H 2) into ethane (C 2H 6). This reaction is commonly used in industry to produce ethane, which is a valuable feedstock for the production of plastics, solvents, and other chemicals.
Structural Formula and Nomenclature
Ethyne has a linear structure with two carbon atoms triple-bonded to each other. The IUPAC name for the product of the complete hydrogenation of ethyne is ethane. The structural formula of ethyne is:“`HC≡CH“`The structural formula of ethane is:“`CH 3-CH 3“`
Mechanism of Hydrogenation
The catalytic hydrogenation of ethyne is a two-step process. In the first step, the triple bond between the two carbon atoms is converted into a double bond by the addition of two hydrogen atoms. In the second step, the double bond is converted into a single bond by the addition of two more hydrogen atoms.The
catalyst for the hydrogenation of ethyne is typically a metal such as palladium or platinum. The catalyst provides a surface on which the hydrogen molecules can adsorb and dissociate into individual hydrogen atoms. The hydrogen atoms then react with the ethyne molecules to form ethane.The
mechanism of the hydrogenation of ethyne can be represented as follows:“`HC≡CH + H 2→ CH 2=CH 2CH 2=CH 2+ H 2→ CH 3-CH 3“`
Physical and Chemical Properties, Draw the product of the complete hydrogenation of ethyne
Ethyne and ethane have different physical and chemical properties. Ethyne is a gas at room temperature, while ethane is a liquid. Ethyne is also more reactive than ethane.The following table compares the physical properties of ethyne and ethane:| Property | Ethyne | Ethane ||—|—|—|| Molecular weight | 26.04 g/mol | 30.07 g/mol || Boiling point |
- 84.1 °C |
- 88.6 °C |
| Density | 1.17 g/L | 1.36 g/L |The hydrogenation of ethyne changes its chemical reactivity. Ethyne is a reactive molecule that can undergo a variety of reactions, including addition, substitution, and cycloaddition reactions. Ethane, on the other hand, is a relatively unreactive molecule.
It can undergo combustion reactions, but it is not as reactive as ethyne.The following are some examples of reactions that demonstrate the different chemical properties of ethyne and ethane:* Ethyne can undergo addition reactions with hydrogen to form ethane.
- Ethyne can undergo substitution reactions with halogens to form haloethanes.
- Ethyne can undergo cycloaddition reactions with dienes to form cyclic compounds.
- Ethane can undergo combustion reactions to form carbon dioxide and water.
Applications
Ethyne is used in a variety of industrial applications. It is used as a fuel, as a feedstock for the production of plastics, and as a starting material for the synthesis of other chemicals.The hydrogenation of ethyne leads to the production of ethane, which is also used in a variety of industrial applications.
Ethane is used as a fuel, as a feedstock for the production of plastics, and as a starting material for the synthesis of other chemicals.Some specific examples of products derived from hydrogenated ethyne include:* Polyethylene
- Polyvinyl chloride
- Ethylene oxide
- Ethanol
- Acetaldehyde
Q&A
What is the IUPAC name for the product of the complete hydrogenation of ethyne?
Ethane
What is the role of the catalyst in the hydrogenation of ethyne?
The catalyst provides an alternative pathway for the reaction, lowering the activation energy and increasing the reaction rate.
What are some industrial applications of ethyne?
Ethyne is used in the production of plastics, synthetic rubber, and other chemicals.